TOP > Report & Column > The Forefront of Space Science > 2015 > The Liquid Boron Turned Out to Be a Semiconductor
![]()

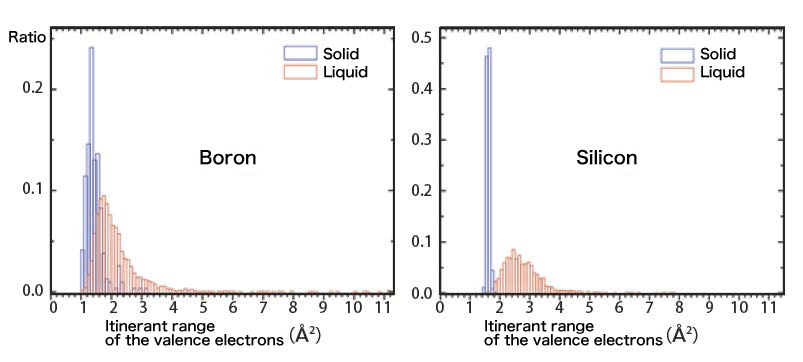
After analyzing the experimental results with a super computer, we were able to visualize the actions of the valence electrons of the boron melt. Figure 4 shows the moving range of the valence electrons. As the horizontal axis goes right, it indicates larger moving range of the valence electrons. For comparison, we put the result of the boron together with that of silicon. The solid silicon is a typical semiconductor with a diamond structure. Since the valence electrons are all imprisoned by the covalent bond between protons, as shown in figure 4, the moving range (itinerant range) of the electrons is limited. When melted, silicon completely turns to a metal, and from the figure we can find that although the electrons in the melt move around freely, the itinerant range has been greatly broadened.
The boron is as well a semiconductor and the valence electrons are imprisoned by the protons, but the crystal structure is much more complicated so there are long covalent bonds in it. Because of this, compared to silicon, the valence electrons of boron have a larger itinerant range. While melted, the distribution of the range showed a shift to the right, but most part of the liquid overlaps with the solid. The boron, being obviously different from silicon, has a similar itinerant range between the liquid and the solid. This indicated that boron is keeping the property of a semiconductor like the solid while melted, without becoming a metal. So far, the boron had been considered to be a metal when melted but in fact, it turned out to be a semiconductor. Heading Towards the Space Experiments On the ground, since we need a high electric field to levitate the sample against gravity, it is necessary to prevent discharging with a high vacuum atmosphere. However, we cannot avoid evaporation and dissociation while heating liquid in a vacuum. Samples that are easy to evaporate or dissociate, such as oxides, which will lose oxygen while heated in a vacuum, will not melt completely. Because of this, there are many substances, such as oxides with a high melting point, the properties of which have not been revealed while in molten state. Meanwhile, in a microgravity environment, since the sample is levitating in the first place, we can control the position of it with a low voltage of several hundred volts. In this case, it will not discharge even in the gas atmosphere. Being able to use gas atmosphere brings great advantages since it is possible for us to melt the materials which cannot be melted down in a vacuum atmosphere. The Electrostatic Levitation Melting Device is going to be launched to the international space station with the H-II Transfer Vehicle ôKounotoriüE5 in the august of this year. We have planned some experiments using the gas atmosphere on ISS to investigate the properties of the materials which are difficult to be melted down in the vacuum atmosphere on the ground. We are going to try our best to make full use of this precious opportunity to obtain new findings in materials. (Junpei Okada, Takehiko Ishikawa)
|
||||||