TOP > Report & Column > The Forefront of Space Science > 2005 > The Science of Containerless Levitation and Supercooled Melts
![]()

Merit of containerless levitation First, containerless levitation allows us to handle such high-temperature liquids that the container materials can not remain solid. Secondly, we can maintain the purity of the liquid sample because there are no impurities included from the container. Furthermore, it is easy to keep liquid samples in a “supercooled state.” 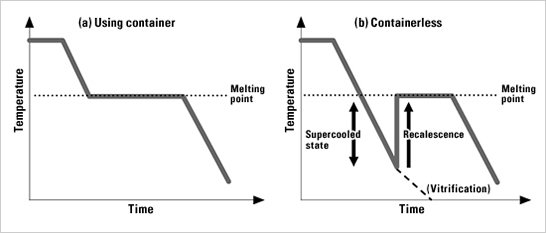
Fig. 2 shows changes in temperature over time as liquid is cooled. When cooled at its melting point in a container, the liquid starts to solidify due to “nuclei” emerging from the container wall. Until solidification is complete, the temperature is maintained by latent heat. Later, after the liquid has completely solidified, the temperature begins to decrease again. Meanwhile, without container, even if the liquid’s temperature goes below its melting point, it remains in a liquid state (i.e., supercooled state) because few coagulation-triggering nuclei are formed. Strictly speaking, even with a container it is possible to produce a slight supercooled state, but the degree of supercooling is very low. Without container, a supercooled state reaching 100 deg. C below melting point is easily produced. When a liquid in supercooled state is cooled further, the liquid usually solidifies because nuclei emerge. In this case, a recalescence is observed, where the sample’s temperature rapidly increases due to the release of latent heat. Sometimes, a supercooled liquid enters a vitrified state directly. In each case, a key feature of containerless levitation is that it can produce a deep supercooled state for an extended period. Using the electrostatic levitation method to measure the thermophysical properties of metals with high-temperature melting points “Physical properties” are the expression in numeric values of a material’s various characteristics, such as density, specific heat and thermal conductivity. The physical properties of water are well known. Most people know some of its properties, such as 1g/cm3 density and 1cal/gK specific heat. The physical properties of metals in solid state have been fully investigated and they are listed in the handbooks. Metals in liquid state, however, show different natures. There are few measurements for liquid metals with a melting point of over 1,000 deg. C. In addition, data fluctuation increases for those metals. This is mostly because of the chemical reaction between the liquid metal and the container. Even for widely used metals such as iron and nickel (with melting points of about 1,500 deg. C), the accuracy of their physical properties in a liquid state is poor. In fact, the accuracy varies more than one order of magnitude compared to that of water. Physical properties in liquid state such as density, specific heat and viscosity of widely used metals, alloys or semiconductors are very important. They are referenced as basic data to determine such manufacturing-process conditions as single-crystal growth of semiconductors, casting, or welding,. Today we can design mold templates via thermal analysis by computer simulations. The accuracy of the simulation is determined by the mathematical model and physical properties. The improvement in accuracy of physical properties still lags far behind, however, compared to the advances in mathematical models used in the computer simulation. In addition, measurement data are very rare for the liquid metals whose melting points are more than 2,000 deg. C, because there are few containers available to withstand such high temperatures. With containerless levitation, we can measure the physical properties of high-temperature liquids which are impossible to measure with conventional methods. Moreover, we can obtain the physical properties in supercooled state. With the electrostatic-levitation method, there is no sample deformation caused by levitation force, and molten samples become spherical even on the ground. This allows us to calculate the volume easily, and also sample density through image analysis. Further, by exciting the drop oscillations, we can measure the surface tension and viscosity of the sample from its oscillation frequency and attenuation time. Fig. 3 shows the measurement results for the molten tungsten, a metal with the highest temperature melting point of approx. 3,420 deg. C. In the past, few of its physical properties have been measured. In particular, there was no data obtained on its viscosity. With our research to date on the ground using the electrostatic-levitation method, we have successfully measured the physical properties of most metal elements with melting points over 2,000 deg. C. 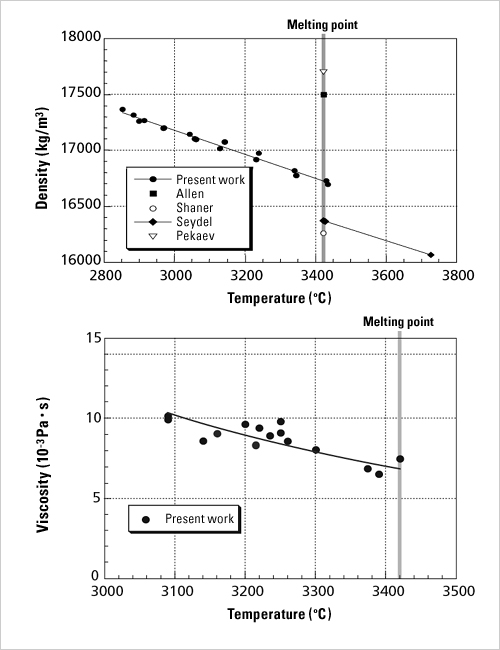
|
||||||




