TOP > Report & Column > The Forefront of Space Science > 2008 > Develop a Low Environmental Impact Propellant
![]()

While previously the term low-pollutionEwas frequently used, today we mostly use low environmental impact.EWhen talking about rockets, the immediate keywords are high-performance,Ehigh-reliabilityEand low cost.EThe keyword low environmental impactEwas also always used in the past, but its priority was low and few took it seriously. However, the situation concerning rocket propellant is now changing. In solid-propellant rockets, ammonium perchlorate (NH4ClO4) is included as an oxidizer and aluminum (Al) as a metallic fuel. When the fuel is burnt, hydrogen chloride (HCl) gas and alumina (Al2O3) particles are emitted. You will intuitively grasp that the oxidizer NH4ClO4 decomposes to generate HCl, and that the aluminum burns (i.e., oxidizes) to become alumina. Hydrogen chloride is prone to combine with hydrochloric acid, and that is known to be harmful to the environment. Although alumina is not basically harmful, it may cause problems because it is released in the form of fine particles. Recently, fine particles called nano-particles have drawn a lot of attention. With few exceptions, most fine particles have a harmful effect on the human body. A close investigation of the hazardousness of alumina fine particles remains to be conducted, but we cannot deny the risk. Since their emission amounts are negligible, questions have not been raised so far. This is not an era, however, where even a small amount can be disregarded. In the current circumstances, we have to be responsible for any environmental impact no matter how small. In addition, strict opinions on alumina particles are now emerging, mainly from Europe, regarding space debris. From a long-term viewpoint, the goal must surely be hydrogen-chloride free in lowerEspace (near to the ground) and fine-particle free in upperEspace (outer space). To this end, one of the following options is necessary:
Option (1) may be possible, but it is out of my scope, and in any case, the complete replacement with liquid rockets is extremely difficult. This article therefore limits discussion to options (2) and (3). Hybrid rocket using GAP The first is the hybrid rocket. There are several types, including the general one that burns fuel polymers using liquid oxidizers (on the premise that the materials used are free of chlorine atoms and metal particles). Various combustion methods have been tried, but the fuel polymers failed to burn as powerfully as expected. Accordingly, we have to find a more powerful polymer. One candidate is a substance called Glycidyl Azide Polymer (GAP). It contains azido group (-N3) in the molecule and the structure is shown in Fig. 1. The azido group is very high-energetic and, when it decomposes to emit nitrogen (N2), it generates extremely high heat. This heat is the power sourceEof GAP. Moreover, it burns by itself without an oxidizer feed. GAP is thus a very valuable polymer. 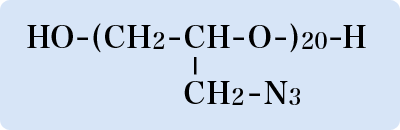
|
||||||||||||




