TOP > Report & Column > The Forefront of Space Science > 2011 > Ever-Evolving Solid Rocket Propellant
![]()

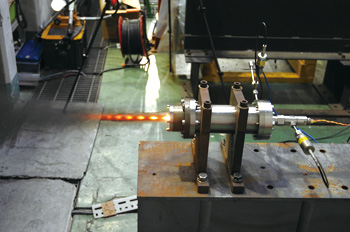
AN (ammonium nitrate) is a crystalline material consisting of H (hydrogen), N (nitrogen), and O (oxygen). AN is widely used as a material for industrial explosives and chemical fertilizer. It is very cheap, about 1/10th of the price of the oxidizer for the solid propellant now used. Although AN has an oxidizer function, its use for the main propulsion system of solid rocket is considered difficult from the viewpoint of combustion performance and physical property. The sub-propulsion system does not require combustion gas as high-temperature as that of the main propulsion system. This is because the sub-propulsion system controls the injection direction of the combustion gas by a movable metal valve. Thus, high-temperature gas exceeding the metalís melting point cannot be used for the system. In fact, the above agent to lower the combustion temperature is used to maintain the gas temperature at about 1,400K. From this, I thought that AN could be used for the GGP. As the first step, we investigated the temperature of combustion gas of solid propellant using AN according to chemical equilibrium computation. We defined HTPB/AP/AN as the basic composition and set the HTPB ratio in the range of 23% to 25% due to ease of manufacture. Then we made the computation by setting the mixture ratio of AN/AP as a parameter. As a result, we discovered that there are combinations whereby the combustion gas temperature becomes lower than 1,400K as shown in Fig. 1. Subsequently, we produced solid propellant in our laboratory to conduct an experimental combustion test using a small rocket motor to confirm whether we could obtain the expected result. Fig. 2 shows a scene of the test. Combustion was stable and we were able to verify that the combustion gas temperature was almost as expected. The result was very helpful in deciding our research direction. 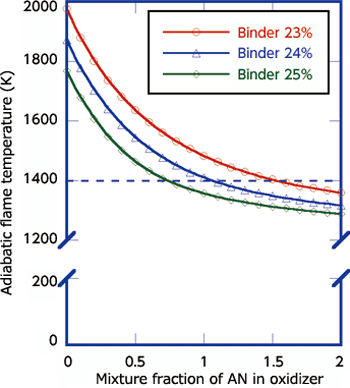
There are several problems to be improved in actual use of the AN-based solid propellant. One is hygroscopicity. For this problem, we plan to introduce new technology in partnership with universities in Japan across different research fields. Research on environmentally friendly solid propellant For those working in the space-related field, the meaning of the term ďenvironmentĀEextends to the range from the ground to orbit in space. Let me introduce our research on environmental-impact reduction of solid propellant from the two standpoints of the ground and in orbit in space. In the solid-rocket propellant, oxygen necessary for combustion is supplied by pyrolysis of oxidizer AP. At the moment, AP is the only oxidizer that is suitable for the manufacture of solid propellant in terms of availability and physical properties. AP is used for solid-rocket propellant across the world, not only in Japan. It contains chlorine atoms (halogens), so the most-generated byproduct of combustion is hydrochloric acid (HCI). Since a large rocket consumes more than 100 tons of solid propellant at a time, a large amount of gas including HCI is emitted around the launch site. Even though the number of rocket launches is still few, the gas surely poses a temporary environmental impact. Therefore, technology to reduce impact on our environment is required. In the meantime, to improve the performance of solid rockets, we must improve the solid propellant by raising the temperature of combustion gas and lowering the average molecular weight of gases generated by combustion. To meet these requirements, we need to discover a chemical that has no halogens, stores much chemical energy, and can be decomposed to much smaller molecules. High Energetic Material (HEM) is a generic name denoting the materials that have some of the properties stated above. In other words, HEM is a storage tank of chemical energy and also has relatively high-thermal energy per unit mass when released by the chemical decomposition process. If material can emit oxygen in the pyrolysis process, it would be very convenient because the material is usable as a high-energy oxidizer. A very important point is that most HEMs do not contain halogens, which is one of APís problems. Therefore, if HEM can be used as an oxidizer of solid rocket, we can realize simultaneously an improvement in propulsion performance of the rocket and reduction of environmental impact.
|
||||||||||




